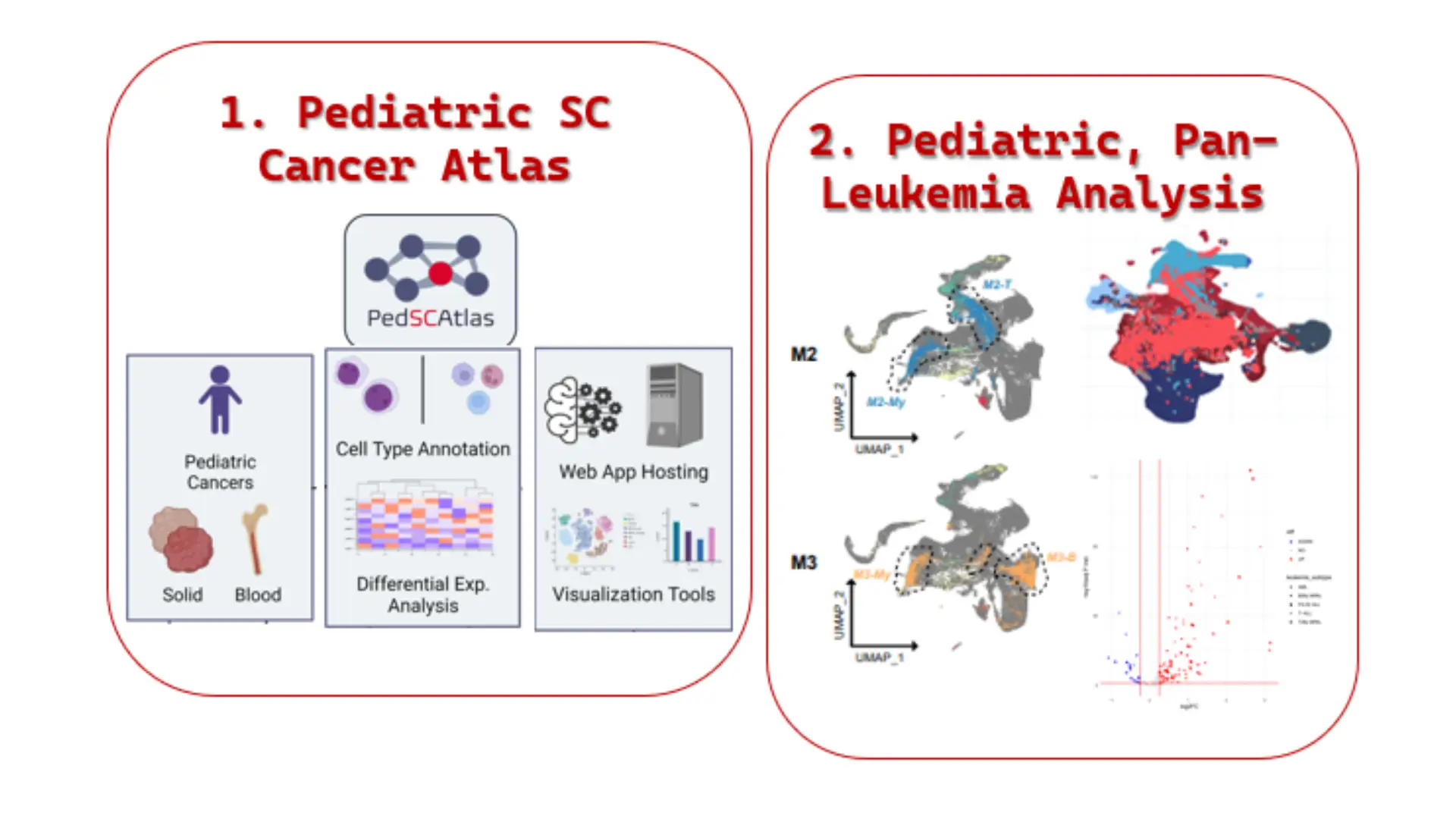
Leukemia is the most common type of pediatric cancer, making up about 24% of new childhood cancer cases. Leukemia cells are malignant, immature blood cells that overpopulate the bone marrow and blood of patients, preventing their bone marrow from producing a sufficient supply of healthy blood cells to maintain their immune system. Acute lymphoblastic leukemia (ALL) is the most common form, followed by acute myeloid leukemia (AML), and the rare mixed phenotype (MPAL). Our lab utilizes omics technologies to analyze acute leukemia at a single-cell level, to develop pan-leukemia and subtype-specific malignant signatures, identify novel drug targets, and understand the pathogenesis of leukemia when compared against healthy bone marrow.
Lab Members Involved:
Relevant Publications
Omics of Obesity in B-cell Acute Lymphoblastic Leukemia
Journal of the National Cancer Institute - Monographs, 2022
Journal of the National Cancer Institute - Monographs, 2022
The obesity pandemic currently affects more than 70 million Americans and more than 650 million individuals worldwide. In addition to increasing susceptibility to pathogenic infections (eg, SARS-CoV-2), obesity promotes the development of many cancer subtypes and increases mortality rates in most cases. We and others have demonstrated that, in the context of B-cell acute lymphoblastic leukemia (B-ALL), adipocytes promote multidrug chemoresistance. Furthermore, others have demonstrated that B-ALL cells exposed to the adipocyte secretome alter their metabolic states to circumvent chemotherapy-mediated cytotoxicity. To better understand how adipocytes impact the function of human B-ALL cells, we used a multi-omic RNA-sequencing (single-cell and bulk transcriptomic) and mass spectroscopy (metabolomic and proteomic) approaches to define adipocyte-induced changes in normal and malignant B cells. These analyses revealed that the adipocyte secretome directly modulates programs in human B-ALL cells associated with metabolism, protection from oxidative stress, increased survival, B-cell development, and drivers of chemoresistance. Single-cell RNA sequencing analysis of mice on low- and high-fat diets revealed that obesity suppresses an immunologically active B-cell subpopulation and that the loss of this transcriptomic signature in patients with B-ALL is associated with poor survival outcomes. Analyses of sera and plasma samples from healthy donors and those with B-ALL revealed that obesity is associated with higher circulating levels of immunoglobulin-associated proteins, which support observations in obese mice of altered immunological homeostasis. In all, our multi-omics approach increases our understanding of pathways that may promote chemoresistance in human B-ALL and highlight a novel B-cell–specific signature in patients associated with survival outcomes.
More information: https://doi.org/10.1093/jncimonographs/lgad014


Relevant Presentations
Single Cell RNA Sequencing Driven Characterization of Pedatric Mixed Phenotype Acute Leukemia
Atlanta Workshop for Single-cell Omics, 2023
Atlanta Workshop for Single-cell Omics, 2023
Background: Pediatric mixed phenotype acute leukemia (MPAL}, a rare subgroup of leukemia, contains features of both myeloid and lymphoid lineage blasts, which makes the disease more difficult to diagnose/treat. More information is needed to understand the origins of the major pediatric MPAL subtypes, B/Myeloid (B/My MPAL) and T/Myeloid (T/My MPAL}, and how they relate to other leukemias. Single-cell RNA sequencing (scRNA-seq) analysis of bone marrow (BM) can provide in-depth information about the leukemia microenvironment and reveal differences/similarities between MPAL subtypes and other types of leukemia that could be exploited to develop novel diagnostics/therapies.
Methods: We analyzed over 40,000 cells from nine pediatric MPAL BM samples to generate the first transcriptomic landscape of pediatric B/My MPAL and T/My MPAL blasts and associated microenvironment cells. Samples collected at the time of diagnosis were used to generate scRNA-Seq data using a droplet-based barcoding technique. After data normalization, clusters were identified using principal component analysis (PCA) and Uniform Manifold Approximation and Projection (UMAP). These unsupervised clusters were used to determine the overall relationship among B/My MPAL, T/My MPAL and other leukemias (acute myeloid leukemia (AML}, B-cell acute lymphoblastic leukemia (B-ALL}, T-cell ALL (T-ALL)). Supervised differentially expressed gene (DEG) analysis was performed to identify B/My and T/My MPAL blast cell signatures. Gene set enrichment analysis (GSEA) was performed, and significantly enriched pathways were compared in MPAL subtypes.
Results: Unsupervised clustering revealed B/My MPAL blasts are more transcriptomically similar to B-ALL and AML blasts, while T/My MPAL blasts are more transcriptomically similar to T-ALL and AML. Subtype DEG analysis of leukemia blasts and healthy BM revealed distinct significantly upregulated gene signatures in B-MPAL (n=35) and T-MPAL (n=17) blasts, which were verified using bulk RNA seq data. Pathway analysis showed upregulated gene activity related to Apoptosis and survival, IL- 8, and S1P2 receptor activation signaling in B/My MPAL blasts. In contrast, S1P2 receptor inhibitory, ERK1/2, and K-RAS signaling were upregulated in T/My MPAL blasts.
Conclusion: Single-cell profiling was used to characterize the molecular landscapes of MPAL blasts and the bone marrow microenvironment and identified gene signatures and pathways that are specifically enriched in B/My and T/My MPAL subtypes.



Investigation of MRD Associated Genes in Pediatric T-ALL Samples at Diagnosis
Atlanta Workshop for Single-cell Omics, 2023
Atlanta Workshop for Single-cell Omics, 2023
Background: T-Cell Acute Lymphoblastic Leukemia (T-ALL}, characterized by the proliferation of immature T cells, accounts for -15% of pediatric leukemias. Minimal residual disease (MRD) assessment after induction and consolidation phases is used to guide treatment decisions. Sustained MRD negativity (MRD-) has shown significant association with continuous remission while higher MRD burden (MRD+} is significantly associated with relapse and poor outcomes in T-ALL. Therefore, we have explored genes associated with MRD outcome in T-ALL bone marrows at diagnosis (Dx).
Methods: Single-cell RNA sequencing (scRNAseq) was performed on viably frozen bone marrow samples provided by the Aflac Leukemia and Lymphoma Biorepository at Children's Healthcare of Atlanta (CHOA) on ten pediatric T-ALL patients at Dx. The scRNAseq libraries were prepared using the 1OX genomics Chromium single cell 3'v3 and S'v1 reagent kits and sequencing was performed using massively parallel sequencing on the Novaseq S4 platform producing >20,000 reads per cell. The single cell count data was normalized using SC Transform function in Seurat v3.0 Bioconductor package.
Results: Out of the ten Dx samples, four were MRD+ and six MRD- after induction therapy. Differential gene expression analysis between the T-ALL blast cells at Dx, showed upregulation of genes such as LGALS1, ITGB1, HSH2D, and CLEC2B in patient samples that are MRD+ relative to those that are MRD-. Some of these genes are associated with chemoresistance, tumor survival, cell adhesion and immune response regulation. Signaling analysis between different cell types such as, NKs, DCs, B & T cells, Monocytes, Erythroid, and TALL blast cells, in the bone marrow microenvironment indicates greater interactions between the different cell types in the MRD subset relative to MRD+ subset. These results suggest that the expression of select genes and signaling interactions in the T-ALL bone marrow at the time of diagnosis indicates association with MRD outcome.
Conclusion: Genes that are associated with MRD can serve as prognostic/ therapeutic targets. By employing insights from systems biology analysis of pathways, networks, and cell-to-cell interactions between leukemia cells and other cells in the bone marrow microenvironment, a robust signature can be established at the time of diagnosis to predict future MRD.












